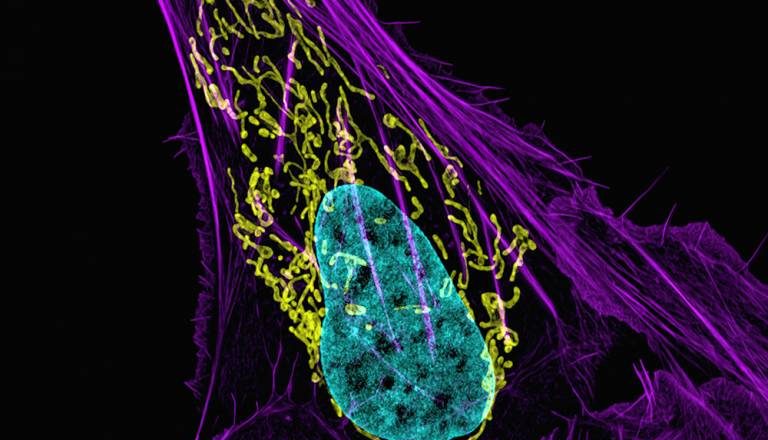
Scientists at UCL have found that a mutation commonly seen in osteosarcomas, the most common type of bone cancer, could render tumours susceptible to a licensed group of cancer treatments known as PARP inhibitors.
Newsletter Signup x
Osteosarcoma is the most common form of bone cancer and disproportionately affects children and young adults. Long term survival in patients diagnosed with osteosarcoma has remained poor compared to other cancers. Although treatments are available, these can cause side effects that may require treatment to be stopped. Crucially, as many as one in three young people diagnosed with osteosarcoma can currently not be cured.
A study led by Professor Sibylle Mittnacht at the UCL Cancer Institute, including researchers from the Crick Institute in London and the IRCCS Istituto Ortopedico in Italy, indicates that a subgroup of osteosarcoma cancers, carrying mutations in the RB1 gene, may be highly sensitive to drugs targeting enzymes known as PARPs. PARP inhibitors are already being used to treat a range of cancers, including ovarian and breast cancers carrying mutations in BRCA genes.
ln laboratory experiments, these so-called PARP inhibitors also had marked effects on the growth of osteosarcoma-derived cancer cells and tumours carrying RB1 mutations. Notably, in side-by-side comparison cells from RB1-mutated osteosarcomas were even more sensitive than cells from BRCA-mutated cancers, where PARP inhibitors are of proven clinical benefit.
RB1 mutations are found in approximately half of osteosarcomas, making it the second most commonly mutated gene in this type of cancer. Importantly, RB1 mutations have been linked to poor prognosis and high risk of metastasis in osteosarcoma.
“As PARP inhibitors are already licensed for clinical use in other cancers, osteosarcoma patients with RB1 mutations could rapidly benefit from these treatments,” says Professor Sibylle Mittnacht, senior author of the study.
The research, published in Nature Communications showed that osteosarcoma cancer cells with RB1 mutation were superbly sensitive to PARP inhibitors, leading to rapid cell death of the cancer cells. These findings extended to osteosarcoma cancer cells that had only recently been derived from a patient’s tumour. The results stood in stark contrast to those obtained with osteosarcoma cancer cells without mutated RB1, including two other osteosarcoma cell models recently derived from patients, which did not respond in this way.
“The fact that the high sensitivity was observed in a cell model that we only recently derived from an osteosarcoma patient is very exciting, and a strong indicator that the sensitivity seen is genuine and unlikely to be due to changes caused by long term growth of cancer cells outside a patient’s body,” says Professor Katia Scotlandi, IRCCS Istituto Ortopedico Bologna, Italy, co-author of the study.
Importantly, when the researchers applied genome engineering to mutate RB1 in osteosarcoma cancer cells in which the gene was not naturally mutated, the cancer cells that previously were unaffected by the PARP inhibitors became highly sensitive and like naturally RB1-mutated osteosarcoma cancer cells underwent cell death. These observations provide direct evidence that the RB1 mutation in cancers is the cause of the inhibitor sensitivity.
Notably, while BRCA-mutated breast and ovarian cancers show distinctive ‘scars’ in their DNA that predict sensitivity to PARP inhibitors, such scars are not seen in RB-mutated osteosarcoma cancer cells or tumours. Therefore, current strategies based on detection of these scars are not a suitable route to predict sensitivity to PARP inhibitors in these cancers.
“The absence of characteristic genomic scaring raises the possibility that another mechanism, distinct from radical homologous recombination DNA repair deficiency that is thought to cause these ‘scars’, may cause hypersensitivity to PARP inhibitors in cancer with RB1 mutation” says Professor Nischalan Pillay, UCL Cancer Institute, and co-investigator on the study.
The work also suggests that a wider range of cancers, including small cell lung cancers and glioblastoma brain cancers where RB1 mutation is observed, may respond to PARP inhibitors, and identifies RB1 mutations as a novel hallmark to recognise tumours likely to be sensitive to these anticancer drugs. PARP inhibitors could therefore be a tailored treatment for patients with other cancers carrying RB1 mutation, apart from osteosarcomas.
“Change in the way osteosarcoma is treated is an urgent requirement to make a difference for patients with this diagnosis. The effects we have seen in our laboratory experiments are very promising. It will now be important that these agents are rapidly tested in clinical trials to confirm their effectiveness in patients”, says Dr Sandra Strauss, University College London Hospital, London Sarcoma Services, who co-led the study.
“Current guidelines on the use of PARP inhibitors are based on characteristics of tumours that are not seen in RB1-mutated osteosarcomas. Using RB1 mutation as a marker of sensitivity to these drugs could open up their use in a much wider range of cancers”, says Dr Zoumpoulidou, UCL Cancer Institute and lead investigator of the study.
“Our findings raise the exciting prospect of a novel, next-generation therapy for a cancer where no progress has been made for more than 30 years”, concludes Professor Mittnacht.
“This discovery from the team at UCL paves the way for more effective treatments for osteosarcoma, a devastating cancer that around 35 children (aged 0-14) are diagnosed with each year. While great progress has been made in recent years for some childhood cancers, osteosarcoma is a long way behind in terms of new treatments and improvements in survival due to a lack of research investment. Current osteosarcoma treatment can sadly have life altering consequences. This new development will hopefully lead to improvements in survival rates and limit long term side effects”, says Christiana Ogunbote, Head of Research, Children with Cancer UK
This study received funding from Children with Cancer UK, Cancer Research UK and the Italian Association for Cancer Research.
to find out more about the research project we helped fund.
Patient Story – Lucie
Lucie was diagnosed with a brain tumour when she was only five weeks old. Her mum Philippa tells their
Read more